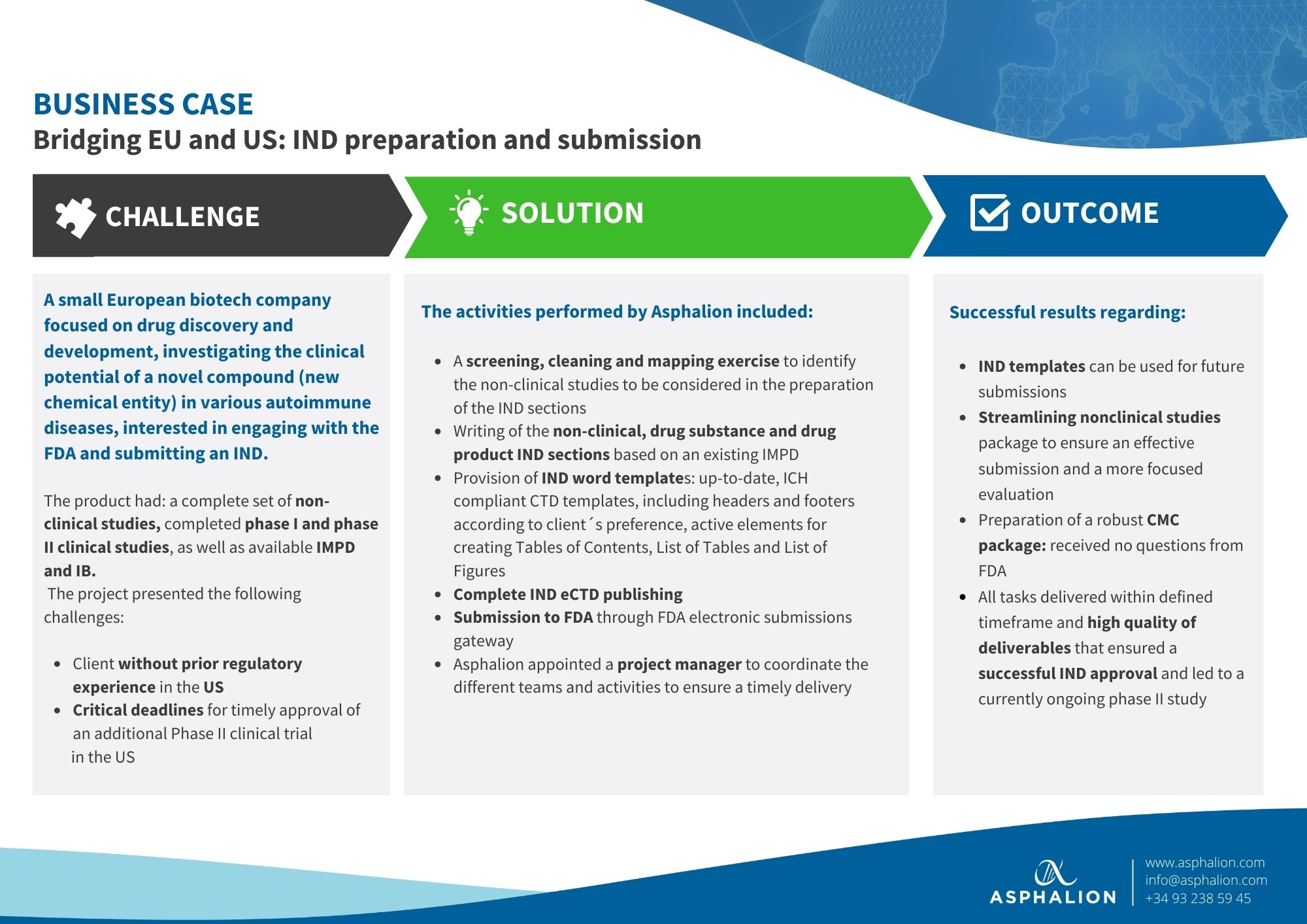
Dealing with FDA can be challenging!!
Have a look at a recent success story of how Asphalion prepared and submitted an IND to FDA for a small European biotech company.
At Asphalion we can assist you with your FDA projects, thanks to our extensive track record of over 20 years.
CLICK HERE TO SEE THE CASE STUDY
For further information you can contact us at: [email protected]