Manufacturers, authorized representatives, system/procedure pack producers and importers of MDR compliant medical devices, as well as notified bodies… ATTENTION!
The European Commission has recently announced a new delay in the entry into force of EUDAMED.
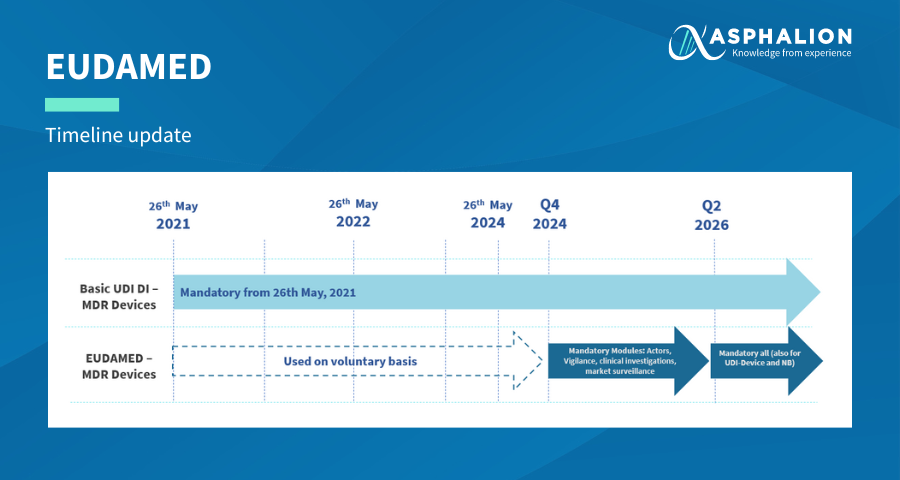
The updated timeline has the following changes:
- The mandatory registration in the actors, vigilance, clinical investigation and performance studies and market surveillance modules is postponed from Q4 2023 to Q4 2024.
- The mandatory registration in the UDI/device and notified bodies and certificate modules is postponed from Q2 2025 to Q2 2026.
In case you need further information, you can contact us at: [email protected]