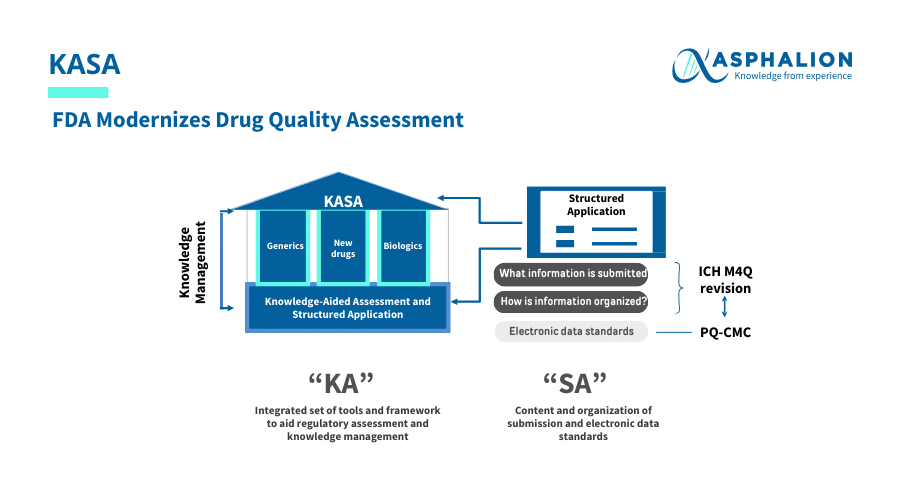
The FDA is transforming drug quality assessments with the new Knowledge-aided Assessment & Structured Application (KASA) system.
This innovative platform captures knowledge throughout a product’s lifecycle, utilizes algorithms for risk assessment and supports computer-aided analysis, promoting efficiency and clarity in the FDA drug approval process.
Key Benefits of KASA:
- Enhances knowledge management and decision-making.
- Streamlines risk assessment and control for products and manufacturing facilities.
- Encourages the use of digital technology to improve regulatory submissions.
ASPHALION CAN HELP WITH:
- Compilation of product quality data in a structured format.
- Support with M4Q guidelines and recommendations when preparing the quality section of the dossier.
- Support with electronic data standards.
- Ensuring
- Updating change management
Contact us! [email protected]