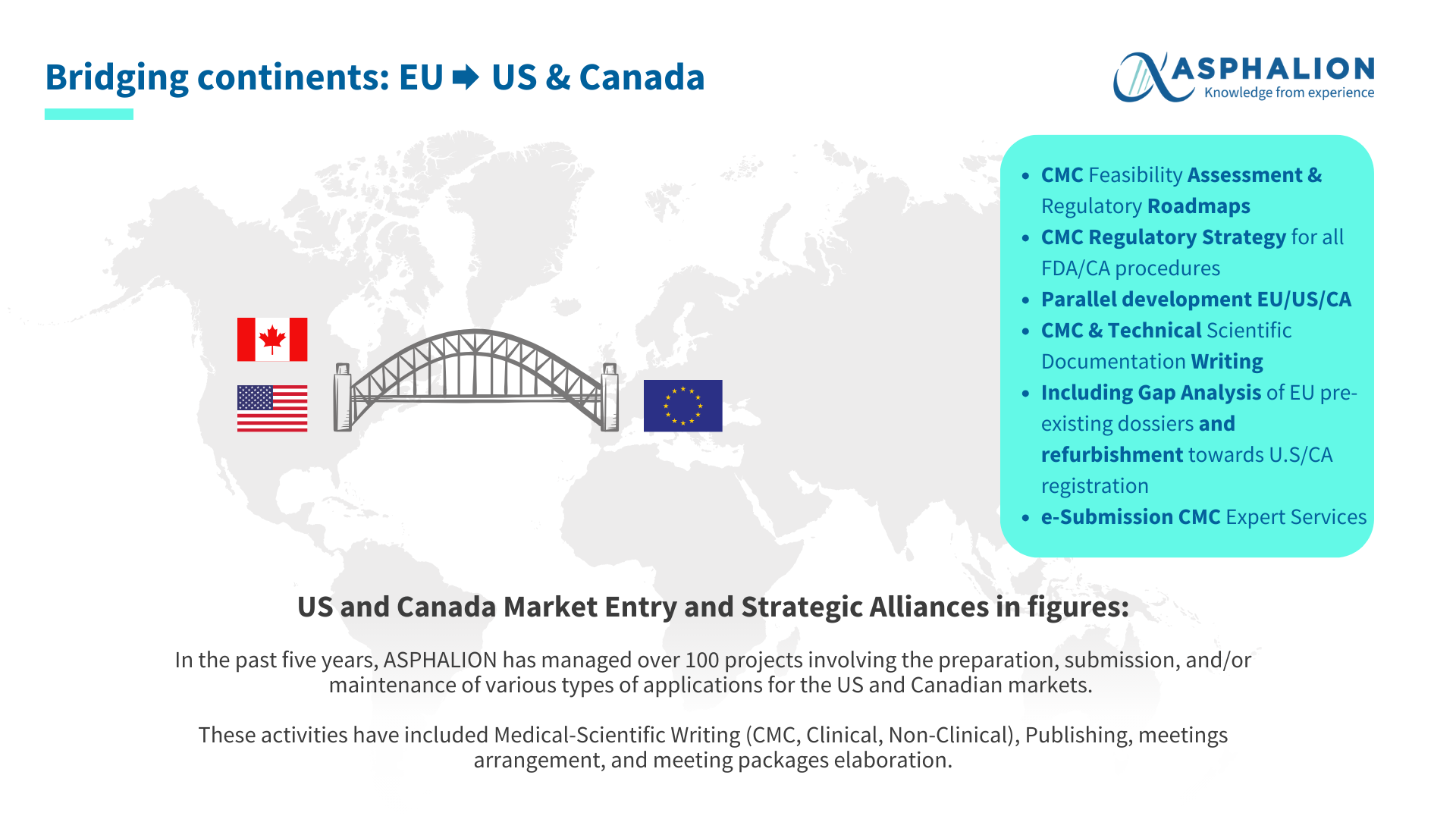
At Asphalion, we are resuming our CMC-week, where we have showcased our expertise in CMC!!
We kicked-off on Monday with a success case where Asphalion helped a biopharmaceutical company with the preparation of an EU IMPD for a biologic product that had started Phase I of a clinical trial in the European Union. On Tuesday we announced the key industry events where Asphalion´s CMC team will be participating. Wednedsday saw our CMC Biologics infographic with all the solutions we can offer during you path from development to market, and beyond! Yesterday, our expert Enrique Solana offered an insightful webinar about the new Certificate of Suitability CEP 2.0. Today, we are wrapping up with our solutions to bridge EU and US & Canada.
Have a look at the image to see how we can help you navigate the complex world of biosimilars with ease! We have extensive experience with preparation, submission, maintenance, etc. of various types of applications in the US & Canada.
Thanks to our extensive track record of over 20 years, at Asphalion we can assist you in selecting the optimal strategy, optimizing costs and enhancing time-to-market, as well as with all other kind of activities and projects.
Contact us today at: [email protected]