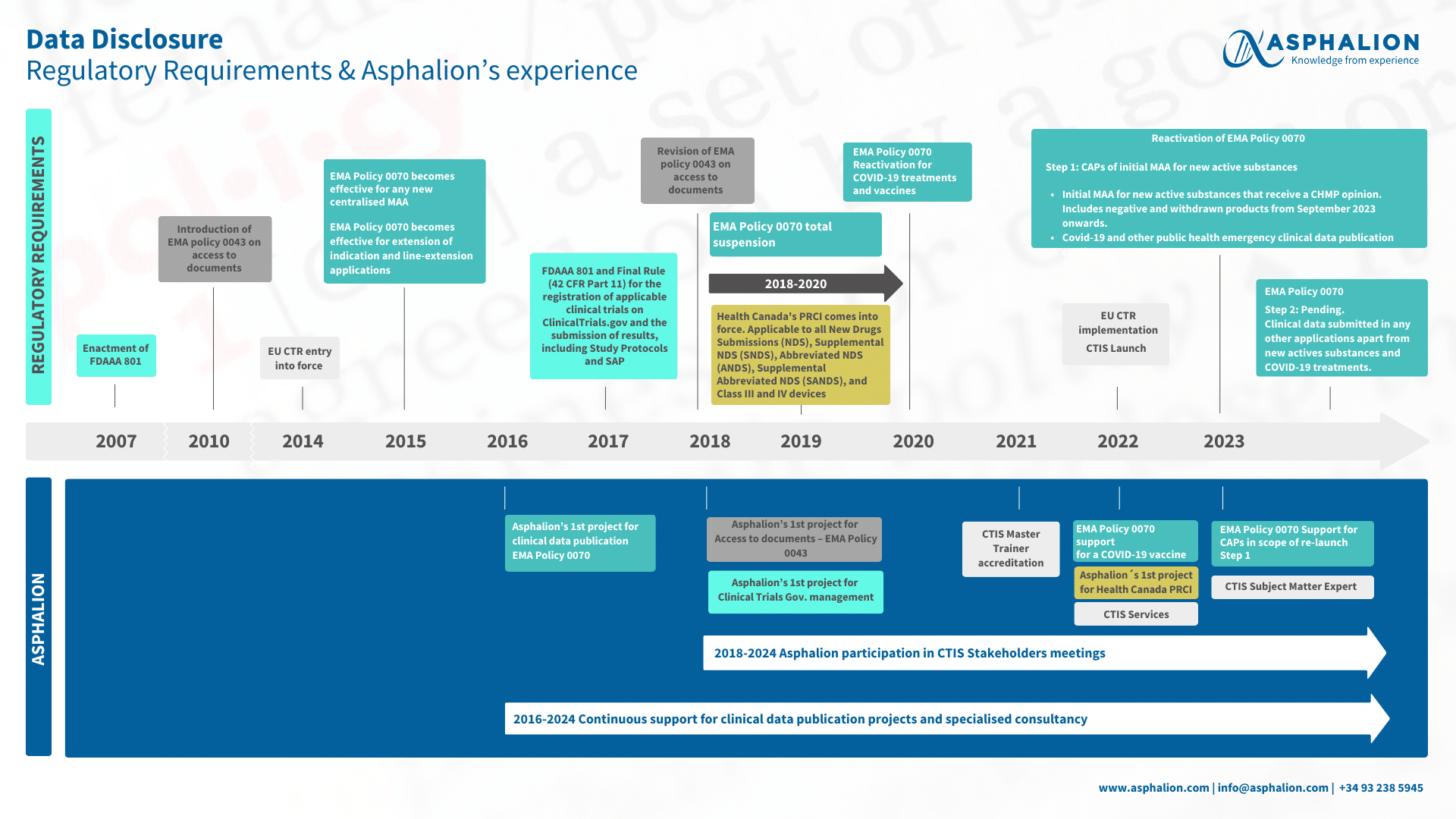
Have a look at this timeline that Asphalion experts have prepared for you to take into consideration about the regulatory requirements for Data Disclosure. You can also discover Asphalion’s expertise on EMA Policy 0070 Policy, EMA Policy 0043, CTIS, etc., linked to EMA requirements.
Have a look at the document here: Data Disclosure Regulatory Requirements
Reach out to us now and learn how Asphalion can help you!
Contact us at: [email protected]