In the dynamic world of veterinary regulation, staying updated is crucial. Don’t get left behind! At Asphalion, we understand the importance of keeping abreast of the latest developments in veterinary regulatory affairs, and we’re here to keep you informed.
Why Staying Updated is Important
The veterinary sector constantly evolves due to scientific advances, technological innovations, and emerging public health needs. Staying informed not only ensures regulatory compliance but also provides companies with a competitive edge by allowing them to anticipate and adapt quickly.
Summary of the Latest Updates
Here is a detailed summary of the latest key updates impacting veterinary regulation:
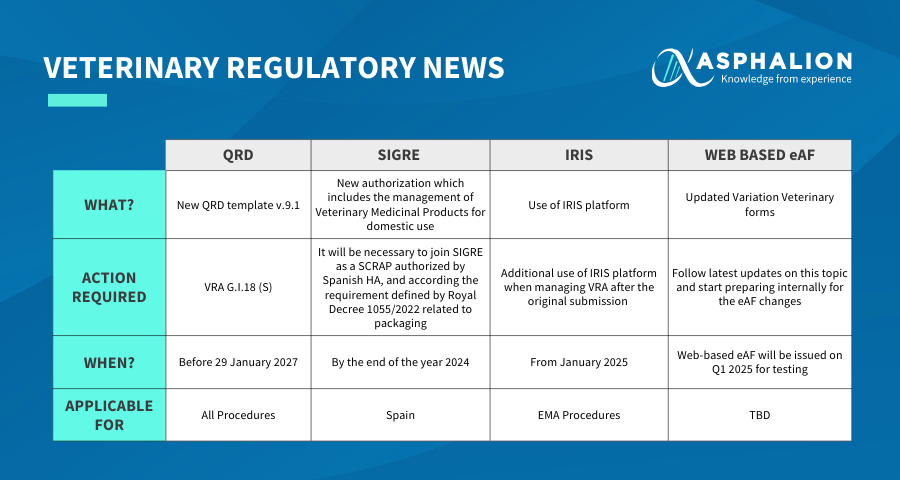
- QRD : A new QRD template v.9.1 requires action (VRA G.I.18 (S)) before January 29, 2027, applicable to all procedures.
- SIGRE : New authorization including the management of Veterinary Medicinal Products for domestic use. Joining SIGRE by the end of 2024 is necessary in Spain as per the Royal Decree 1055/2022 related to packaging.
- IRIS : Usage of the IRIS platform will be essential from January 2025 for EMA procedures, especially after the original submission.
- WEB-BASED eAF : Updated Variation Veterinary forms will be issued for testing in Q1 2025. It’s essential to prepare internally for these changes.
How We Can Help
If you have any questions or need further clarification, don’t hesitate to reach out. Our team of experts is here to help you navigate these complexities and ensure compliance with all relevant regulations.
Contact us at: [email protected]