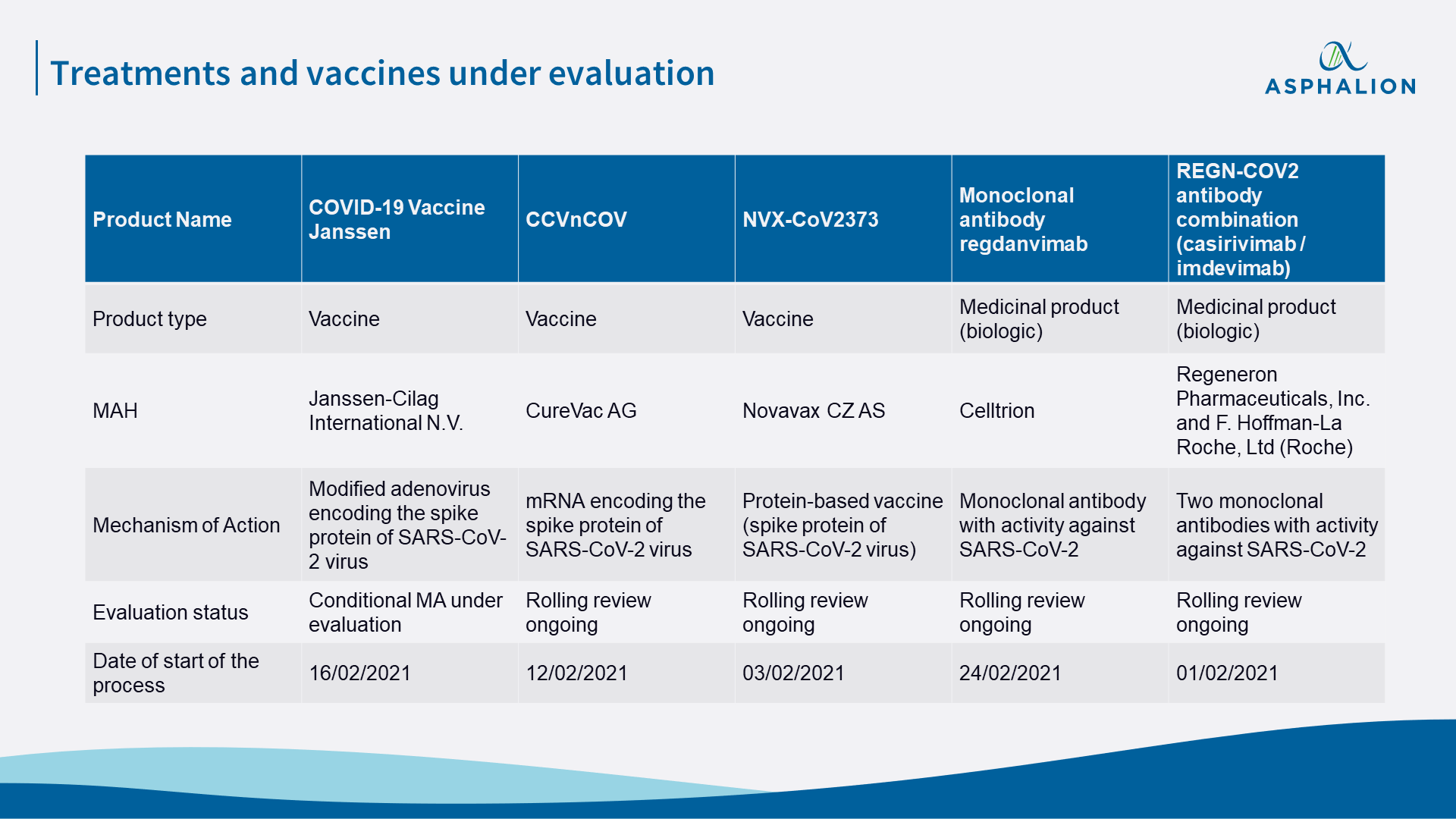
Two days ago, EMA notified the start of a rolling review of data on the monoclonal antibody regdanvimab (Celltrion) for the treatment of COVID-19. These are great news, as now there are a total of 5 products being evaluated by EMA (3 vaccines and 2 treatments) which could be placed on the market during the following months, depending on the results of the evaluation.
The development of new tools to treat COVID-19 brings renewed hopes to fight against the pandemic. At Asphalion we are actively aiding in the development of vaccines and treatments for COVID-19, bringing our best to help all developers get their products ready to be authorized.
For further information you can contact us at: [email protected]